NAFDAC Alerts Nigerians Over Suspected Fake Augmentin
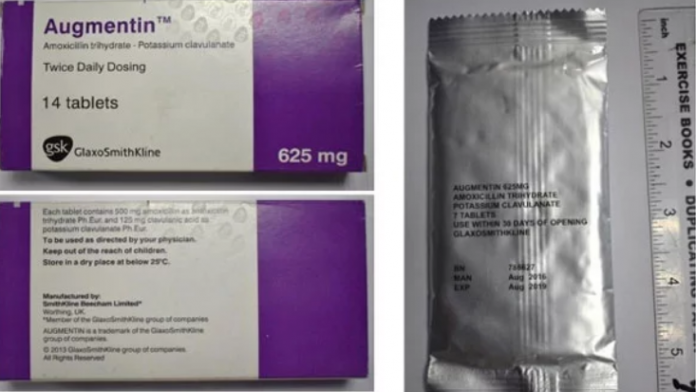
The National Agency for Food and Drugs Administration and Control (NAFDAC) has raised the alarm concerning the circulation of suspected counterfeit Augmentin 625mg Tabs in Nigeria.
According to NAFDAC, the product failed short of the labeling requirements.
The alert signed by the management of NAFDAC and titled ‘NAFDAC Alert And Sensitization Of The Public On Suspected Falsified Augmentin 625mg’ was posted on the agency’s website.
NAFDAC said the suspected falsified Augmentin which was manufactured in April 2021 would expire on April 2024.
It added that the batch number of the falsified drug is 562626 with a NAFDAC registration number- 04-1928.
It said “The product failed short of the labeling requirements. No inscription “manufactured by” is written on the label -only the address.
“Manufacturing and Expiry dates do not meet the acceptable format. No MAS scratch number for verification.
“The logo “gsk” is not properly positioned as on the original. The listed information indicates the product is falsified and counterfeited.
“In view of the above, NAFDAC has notified all its formations in the zones and 36 states of the federation including the FCT to carry out surveillance and mop up the falsified Augmentin tablets.”
“Please note that the genuine Augmentin 625mg has legible product labeling information including date markings – expiration and manufactured dates, batch number and NAFDAC registration number.
“NAFDAC advise to wholesalers, distributors, and pharmacies is that medicines should be obtained from authorized/licensed suppliers, increased vigilance is hereby encouraged within the supply chain to avoid infiltration of the falsified product. The products’ authenticity, physical condition and labels should be carefully checked.
“NAFDAC implores healthcare providers to ensure vigilance to prevent the administration of falsified products to unsuspecting patients. Members of the public in possession of the above suspected counterfeit product are advised to discontinue sale or use and submit stock to the nearest NAFDAC office”.



